Styrene: Issues and implications
The facts (such as they are) behind the furor surrounding this vital crosslinking chemical’s listing in the 12th RoC.
Among the chemicals used to manufacture polymer composites, few are as widely used as styrene. Produced from natural gas feedstocks, it is a primary ingredient in polystyrene (PS), acrylonitrile butadiene styrene (ABS), synthetic rubber, paints and, most important to the composites industry, expanded polystyrene (EPS) and styrene acrylonitrile (SAN) used to make foam core, and unsaturated polyester resin (UPR).
In UPR, styrene is used in loadings up to 40 percent by weight and plays a key crosslinking role during cure. UPR represents the second-largest global market for styrene (behind PS), and in the composites industry UPR is a workhorse. Its corrosion resistance and high strength have long made it popular in the manufacture of marine watercraft, cultured marble countertops, solid-surface bath and kitchen ware, polymer concrete and more. Most composite boats, for example, are still manufactured via open molding processes (bucket and roller, or spray up). That and its strong odor have kept styrene under the wary eye of government and regulatory agencies as a substance potentially harmful to employees in composites manufacturing facilities.
Since the 1980s, in fact, a variety of state, national and international organizations have assessed the health effects of styrene and deemed it not harmful to humans if managed using the accepted guidelines. However, in 2004, the U.S. National Toxicology Program (NTP), under the U.S. Department of Health and Human Services (HHS), received a recommendation from an unidentified private individual to assess the health effects of styrene. The resulting decision, in June 2011, was to list styrene in the 12th Report on Carcinogens (RoC), officially labeling it as “reasonably anticipated to be a human carcinogen.” Chemical and composites industry trade associations responded swiftly, petitioning the Obama Administration and HHS Secretary Kathleen Sebelius to delist styrene.
Although the NTP lacks direct regulatory authority — that is, the listing does not preclude styrene’s use, nor does it necessarily lead to greater restrictions in terms of its use — the RoC is a nationally recognized, often-referenced document that insurance companies use to justify either increased rates or termination of coverage. For composites manufacturers that use UPR, therefore, the immediate effects are financial.
Styrene history
Styrene, a/k/a ethenylbenzene or vinyl benzene, is an organic compound represented by the chemical formula C6H5CH=CH2. First isolated in 1831 by distillation of storax, a natural balsam, styrene’s commercial production began in 1925 in Germany. About 15 billion lb (6.8 million metric tonnes) of styrene is produced globally today.
The two materials that most depend on styrene are PS and UPR. PS was discovered in 1839, but it wasn’t until 1931 that I.G. Farben (Ludwigshafen, Germany), with the help of newly available large quantities of styrene, began earnest production. UPR was first prepared in 1847, but it wasn’t produced on a large scale until the 1930s, when new chemistries were developed and styrene became readily available. UPR has been used since the 1940s to mold a variety of composite parts and structures.
Styrene is highly flammable, and readily polymerizes when exposed to light or heat. It enters the human body primarily via inhalation, although transmission via skin contact is possible.
Currently there is no chemical alternative that can facilitate UPR crosslinking as effectively and inexpensively as styrene does, and until one is developed, UPR will remain styrene-dependent.
Because UPR has been in use for 60 years, thousands of employees who work in styrene-rich environments have been studied for the effects of the chemical. These studies allow scientists to assess the likelihood that styrene exposure could cause cancer in humans.
Styrene exposure
Human studies of styrene’s health effects focus primarily on the duration and concentration of exposure, typically measured in parts per million (ppm). Animal studies of styrene’s health effects have been conducted primarily with mice and rats.
The composites manufacturing industry, because of open molding operations, provides the greatest occupational exposure to styrene. In general, occupational styrene exposure of about 200 ppm was the norm in the 1940s through the early 1960s, but as ventilation and respiratory protection practices improved, and as new resin systems and molding technologies were developed, exposure trended down toward 20-100 ppm in Europe and 50 ppm in the U.S.
According to the International Agency for Research on Cancer (IARC, Lyon, France), the average exposure to styrene in open-mold processes is two to three times greater than in press-mold processes. A variety of studies in Europe and the U.S. show that chopper gun operators have the highest exposure, followed by laminators and gel coaters. Further, the IARC notes that wearing a respirator suitable for organic vapors markedly reduces styrene exposure but does not eliminate it entirely.
In 1989, the U.S. Occupational Safety and Health Admin. (OSHA) established a safe-exposure standard for styrene of 50 ppm over an eight-hour day. In 1992, a U.S. appeals court voided that limit, thus reinstating the pre-1989 limit of 100 ppm. The U.S. National Institute for Occupational Safety and Health (NIOSH) specifies a 100-ppm short-term exposure limit (STEL) and a 50-ppm recommended exposure limit (REL). Starting in 1997, the U.S. styrene and composites industry asked their members comply with an eight-hour average occupational exposure limit of 50 ppm, to protect against nervous system effects such as temporary drowsiness.
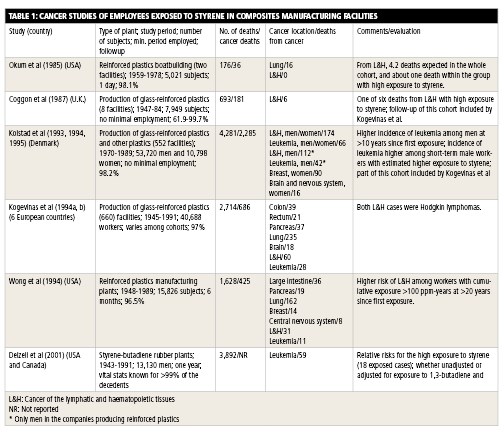
Cancer studies
Cancer studies of workers exposed to styrene in composites manufacturing facilities typically report the number of subjects (people) in the population studied, time period studied, total number of deaths in the population, total number of deaths from cancer in the population, types of cancer detected and, occasionally, the number of cancers and deaths expected in the population. These values are measured, and study authors attempt to determine if there is a statistically significant correlation between styrene exposure and incidence of cancer. In other words, is the incidence of cancer in a population directly attributable to that population’s exposure to styrene? If so, what levels of exposure produce this correlation?
Most cancer studies were conducted in Europe and the U.S. The studies are too numerous to summarize here, but the largest and most notable are worth reviewing (see below).
The largest and most comprehensive cancer study of composites industry workers exposed to styrene, by H.A. Kolstad et al., evaluated 53,720 men and 10,798 women at Danish facilities between 1970 and 1989. There were 4,281 deaths, 2,285 of which were caused by cancer. The cancers included lymphocytic and histiocytic tumors, leukemia, breast cancer and brain cancer. The mean annual styrene exposure was 180 ppm from 1964 to 1970 and 43 ppm from 1976 to 1988. The study found that there was a statistically significant increased risk for leukemia 10 years after initial employment, but only in workers employed for less than a year. The authors said that they did not believe these data support a cancer concern for styrene.
Another major study, by M. Kogevinas et al., included 40,688 workers employed at 660 plants in Denmark, Finland, Norway, Sweden and the U.K. from 1945 to 1991. There were 2,714 deaths, 686 of which were from cancer, including lung, lymphatic and hematopoietic, colon, pancreatic, rectal, and brain cancer, as well as leukemia. The exposure to styrene in Denmark, which is considered representative for the study, was 200 ppm in the early 1960s, 100 ppm in the late 1960s and 20 ppm in the late 1980s. Given the incidence of these diseases in the general population, study authors did not believe their findings support a link between styrene and cancer.
O. Wong et al. studied 15,826 male and female employees who worked at 1 of 30 composites facilities in the U.S. for at least six months between 1948 and 1977. The workers were followed until 1989. The study reported 1,628 deaths overall, 425 of which were from cancer. Reported cancers included bronchus/trachea/lung, large intestine, lymphatic and hematopoietic, pancreatic and breast, as well as leukemia and cancers involving the central nervous system.
One study referenced in the decision to list styrene in the 12th RoC was led by Dr. Elizabeth Delzell, an epidemiologist at the University of Alabama – Birmingham. The study did not focus on styrene exclusively, but on the use of styrene-butadiene in the manufacture of synthetic rubber. A total of 15,649 male employees who worked for at least one year at one of eight synthetic rubber plants in the U.S. were studied from 1943 to 1991. The overall styrene exposure in this group was lower than the average exposure in a composites manufacturing facility. Still, the study showed an apparent correlation between styrene exposure and risk of leukemia. However, the presence of 1,3-butadiene in the exposure models made it difficult to determine precisely where styrene’s effects ended and 1,3-butadiene’s effects began.
The only animal testing has been conducted on mice and rats that were exposed via ingestion and inhalation. The ingestion data showed no clear correlation to the development of cancer. However, both male and female mice exposed to various concentrations of styrene vapor showed a statistically significant increase in lung cancer development.
Based on these and other studies, a variety of regulatory bodies in Europe and the U.S. have weighed in on the health threats posed by styrene. The data have been interpreted differently on both sides of the ocean.
The decision to list
Before a chemical was listed in the 12th RoC, it first was nominated to the NTP. After that, the NTP employed a complex review process to assess the human and animal health risk data associated with the chemical. To aid the NTP in its assessment, a peer review panel of experts on the chemical in question was convened. This panel helped the NTP interpret scientific data and made a listing recommendation to the NTP. Since the 1st RoC was published in 1980, 240 substances (or classes of related substances) have been listed, and three substances were reviewed but were not listed. Dr. Ruth Lunn, NTP director of Office of the Report on Carcinogens, says the NTP more often than not lists a chemical once it’s nominated and notes that the NTP makes the final listing recommendation to the Secretary of the Department of Health and Human Services as to whether or not a chemical is listed. For the 12th RoC, the NTP, she says, generally followed the recommendation of the peer review panel.
If a chemical is listed in the RoC by the NTP, it is listed either as “known to be human carcinogen” or, in styrene’s case, “reasonably anticipated to be human carcinogen.” The “reasonably anticipated” status, the NTP says, requires “limited evidence of carcinogenicity from studies in humans, which indicates that causal interpretation is credible, but that alternative explanations, such as chance, bias, or confounding factors, could not adequately be excluded; or, there is sufficient evidence of carcinogenicity from studies in experimental animals, which indicates there is an increased incidence of malignant and/or a combination of malignant and benign tumors (1) in multiple species or at multiple tissue sites, or (2) by multiple routes of exposure, or (3) to an unusual degree with regard to incidence, site, or type of tumor, or age at onset ... or there is convincing relevant information that the agent acts through mechanisms indicating it would likely cause cancer in humans.”
Although NTP criteria are written as specifically as possible, scientists and analysts often look at the same data regarding a material’s toxicity and reach different conclusions about what they mean. “There is no preconceived philosophy [at the NTP],” says Lunn. “Our goal is to look at the science and apply the criteria.”
Summarizing succinctly all of the reasons that the NTP chose to list styrene in the RoC is nearly impossible (but see “Learn More” for online assistance toward that end). But it can be noted here that the NTP listed the styrene-butadiene data in the Delzell study, leukemia risk in the Kolstad study, DNA damage in rat studies and lung tumors in the mouse study, among other correlations as the reasons for its listing. In the 12th RoC, the NTP also emphasizes immediately that its decision is based in part on its “limited evidence” standard: “The limited evidence for the carcinogenicity of styrene in humans is based on studies of workers exposed to styrene that showed (1) increased mortality from or incidence of cancer of the lymphohematopoietic system and (2) increased levels of DNA adducts and genetic damage in lymphocytes from exposed workers.” The RoC also says: “Studies in the reinforced-plastics industry provided evidence that suggests a possible association between styrene exposure and cancer of the esophagus or pancreas.” And: “DNA adducts … were found in circulating white blood cells in many studies of styrene-exposed workers employed mainly in the reinforced-plastics industry.”
Disagreement
Among the most critical of NTP’s decision to list styrene in the RoC has been Gradient Corp. (Cambridge, Mass.), an environmental and risk science consulting firm that specializes in toxicology, epidemiology and risk assessment. Gradient was a commenter to the NTP when the draft assessment of styrene was first published, and more recently has written a peer-reviewed article for the journal Human and Ecological Risk Assessment, titled, “The Weight of Evidence Does Not Support the Listing of Styrene as ‘Reasonably Anticipated to be a Human Carcinogen’ in NTP’s Twelfth Report on Carcinogens.” Gradient argues that the NTP found evidence of styrene-caused cancer where none exists: “The epidemiology data as a whole do not suggest that styrene exposure is associated with any specific cancer type in humans, either within or among studies. The NTP profile, however, interprets these data as suggesting that styrene exposure increases the incidence of lymphohematopoietic cancers, and possibly pancreatic and esophageal cancers, in humans (NTP, 2011). Even if one accepts this interpretation, there have been no corresponding responses observed in experimental animals, as no increased incidences of lymphohematopoietic, pancreatic, or esophageal tumors have been reported in styrene-exposed animals. The experimental animal data also indicate that orally administered styrene does not induce tumors systemically; although the data are unclear, in some studies with inhalation and oral exposures, an increased incidence of tumors was observed specifically in the mouse lung.”
In particular, Gradient notes that evidence of cancer in the mouse lung is insufficient as a potential cause of cancer in humans. “In summary, there is evidence that styrene causes an increased incidence of lung tumors in mice after inhalation exposure. Other studies in mice that used oral gavage as the exposure route were equivocal, and tumor incidence was not increased in one study that used intraperitoneal injection. Thus, an increased tumor incidence caused by styrene exposure has only been observed in one experimental animal species and at one tissue site.”
The NTP heard these arguments during its public comment period before the listing decision was made and had this response: “The available epidemiological data have not found evidence that styrene causes lung cancer in humans, although most studies were limited by short follow-up and lacked detailed analyses for lung cancer. However, the human epidemiological studies do provide limited evidence [italics added] that styrene causes lymphohematopoietic cancers. The induction of lung tumors in mice and lymphohematopoietic cancers in humans has also been observed in studies of exposure to epoxides and other epoxide-forming chemicals including the known human carcinogens 1,3-butadiene and ethylene oxide (NTP, 2004).”
Others have chimed in on styrene as well. One is Paolo Boffetta, director of the Institute for Translational Epidemiology at Mount Sinai School of Medicine (New York, N.Y.), who led a group of scientists that published “Epidemiologic Studies of Styrene and Cancer: A Review of the Literature” in the Journal of Occupational and Environmental Medicine (November 2009). Funded by a grant from the Styrene Information and Research Center (SIRC, Arlington, Va.), the review concludes, “The evidence for human carcinogenicity of styrene is inconsistent and weak. On the basis of the available evidence, one cannot conclude that there is a causal association between styrene and any form of cancer.”
Boffetta adds: “There are, however, steps that could be undertaken to better exploit the available epidemiologic data. First, the follow-up of the two largest studies of reinforced plastic workers should be updated since 15 or more additional years of mortality experience would be available. Second, information should be obtained on NHL [non-Hodgkin lymphoma] subtype of cases in the most informative cohorts. Third, a pooled analysis of studies of reinforced plastic workers should be considered in order to increase statistical power, to eliminate overlaps between studies, and to provide results according to comparable exposure categories.” American Composites Manufacturers Assn.’s (ACMA, Arlington, Va.) senior director for government affairs, John Schweitzer, notes that all of these suggested studies are completed or underway.
In a February 2009 letter to the NTP’s Board of Scientific Counselors, Delzell, in response to the NTP’s Draft Substance Profile for styrene, pointed out flaws in some of the conclusions made by the NTP regarding carcinogenicity and concluded, “The available scientific evidence is not sufficient to conclude that styrene causes lymphoma, leukemia or other cancers. In particular, the lack of consistent, reasonably precise associations between estimated exposure to styrene and NHL or leukemia in the studies of reinforced plastics industry workers is an important shortfall of the evidence for the hypothesis that styrene causes these cancers.”
What others are saying
The ACMA has been particularly critical of the 12th RoC listing. ACMA’s Schweitzer has been the face and voice of the group’s effort first to keep styrene off the list, and now to have it removed. His concerns about how the NTP handled the styrene nomination revolve around what he calls the application of “bad policy leading to bad science.” He believes the NTP’s styrene expert panel was predisposed to list styrene as a carcinogen and, therefore, interpreted the data to meet that expectation. “Scientists often disagree about statistical analysis, and that’s fine,” he says. “But this is a group adept at changing definitions on the fly to meet their needs.” Further, Schweitzer believes that the NTP followed nonscientific processes as it considered the evidence. For example, he points to the studies in which mice developed lung cancer following styrene exposure, but rats and humans did not. He argues that the best data suggest that styrene in the lungs of a mouse is metabolized into 4-vinylphenol, which is, in turn, further metabolized into dihydroxystyrene or another highly toxic substance. By contrast, in the lungs of humans and rats, styrene is metabolized into styrene oxide, which is further metabolized into a variety of nontoxic substances. Further, he contends that in the NTP review process “any data and any theory supporting a cancer concern is considered plausible no matter how weak or inconsistent.” He says the process does not make proper use of peer review and does not allow meaningful response to outside scientific input.
Similarly, Joe Walker, communications advisor to the SIRC, says his organization is looking for process fairness and consistency in the risk evaluation process. “The science does not support the listing and the NTP does not use a process that supports that listing,” he says.
NTP’s Lunn noted repeatedly to CT that the peer review panel and the NTP are guided exclusively by the listing criteria and that there is no other guiding philosophy or ideology in use.
In Europe, the IARC has its own hierarchy of carcinogenicity that it applies to chemicals: 1A (most carcinogenic); 2A (probably carcinogenic); 2B (possibly carcinogenic); 3 (not classifiable as to its carcinogenicity); 4 (probably not carcinogenic). The IARC classified styrene as 2B. The European Union also has a styrene health-effects dossier included in its Registration, Evaluation, Authorisation and Restriction of Chemical substances (REACh) program that proposes styrene should not be classified as a carcinogen at all.
What happens now?
Since publication of the 12th RoC, the ACMA has led a lobbying effort, calling for re-evaluation of the data and removal of styrene from the report. A letter signed by 63 Congressional representatives was sent to HHS in May 2011. In November 2011, 10 senators and 50 representatives sent a letter to HHS asking that the National Academy of Sciences (NAS) be allowed to review the 12th RoC. The U.S. Small Business Admin. (SBA) also sent a letter to HHS, asking Secretary Sebelius if the RoC “should continue to play a role in the federal government’s chemical risk assessment program.” In September 2011, 21 member companies of the ACMA sent a letter to White House chief of staff William Daley, asking that President Obama direct the NAS to study the “link between human cancer and styrene exposure.” In December 2011, the U.S. Congress allotted funding in its 2012 appropriations for a scientific peer review by the NAS of the RoC listing.
In the meantime, the SIRC has filed suit against HHS, seeking a legal injunction to withdraw the 12th RoC as it relates to styrene. Although legal hearings have been held in this case, it has not yet gone to trial.
Lunn says that under current NTP policy, delisting a material from the RoC is possible, but doing so requires new scientific data that, when evaluated under the NTP’s criteria, is sufficient to remove the chemical as a carcinogenic threat. “If you want to nominate a material for delisting, which can be done at any time, there must be new data,” she says. Since 1980, eight chemicals have been delisted from the RoC, the last one being Saccharin, in 1998. Lunn could not comment on the possibility of a delisting of styrene via political or other avenues.
It remains to be seen how, over the long term, the listing in the 12th RoC will impact styrene use. It is possible that those who market styrene-containing products will be required to label them with a toxicity warning. OSHA and other regulatory agencies might also use the RoC listing to justify new or modified guidelines regarding styrene exposure and use.
Further, ACMA says some of its member companies already have reported loss of insurance coverage or premium increases because of increased liability caused by the RoC listing. “Insurance companies don’t care if it’s a scientifically valid process,” says Schweitzer. “If they’re sued, it costs them money.” Schweitzer also reports that some composites education programs, worried about their liability, are limiting or discontinuing some courses.
Web sites of the groups mentioned in this article are as follows:
SIRC: www.styrene.org
NTP: ntp.niehs.nih.gov
IARC: www.iarc.fr
ACMA: www.acmanet.org
EPA IRIS: www.epa.gov/iris
Documents from the NTP and European organizations regarding the listing of styrene in the RoC.
Boffetta assessment
Danish EPA styrene review
European styrene industry review
Elizabeth Delzell letter to NTP regarding styrene
IARC styrene monograph
NTP styrene fact sheet
NTP styrene panel report A
NTP styrene panel report B
NTP panel peer review response
NTP panel response to public comments
NTP styrene in 12th RoC
Letter to Composites Technology from the Styrene Information Research Center following publication of the above story:
Related Content
PEEK vs. PEKK vs. PAEK and continuous compression molding
Suppliers of thermoplastics and carbon fiber chime in regarding PEEK vs. PEKK, and now PAEK, as well as in-situ consolidation — the supply chain for thermoplastic tape composites continues to evolve.
Read MorePlant tour: Albany Engineered Composites, Rochester, N.H., U.S.
Efficient, high-quality, well-controlled composites manufacturing at volume is the mantra for this 3D weaving specialist.
Read MoreThe potential for thermoplastic composite nacelles
Collins Aerospace draws on global team, decades of experience to demonstrate large, curved AFP and welded structures for the next generation of aircraft.
Read MoreMFFD thermoplastic floor beams — OOA consolidation for next-gen TPC aerostructures
GKN Fokker and Mikrosam develop AFP for the Multifunctional Fuselage Demonstrator’s floor beams and OOA consolidation of 6-meter spars for TPC rudders, elevators and tails.
Read MoreRead Next
Developing bonded composite repair for ships, offshore units
Bureau Veritas and industry partners issue guidelines and pave the way for certification via StrengthBond Offshore project.
Read MoreAll-recycled, needle-punched nonwoven CFRP slashes carbon footprint of Formula 2 seat
Dallara and Tenowo collaborate to produce a race-ready Formula 2 seat using recycled carbon fiber, reducing CO2 emissions by 97.5% compared to virgin materials.
Read More“Structured air” TPS safeguards composite structures
Powered by an 85% air/15% pure polyimide aerogel, Blueshift’s novel material system protects structures during transient thermal events from -200°C to beyond 2400°C for rockets, battery boxes and more.
Read More
















.jpg;maxWidth=300;quality=90)