When optimized and verified through reliable testing, plasma is an effective way to change the chemical composition of the surface of a material that is to be bonded. Source, all images | BTG Labs
Surface treatment — involving some method of treating, abrading or cleaning a part or material surface — can be essential to achieving the necessary properties for successful adhesive bonding, coating or even painting. However, some methods are more effective than others for certain materials.
According to Giles Dillingham, CEO and chief scientist at BTG Labs (Cincinnati, Ohio, U.S.), surface treatment of materials for bonding, coating or sealing needs to accomplish three things:
- Cleaning: This means reducing the amount of detrimental contaminants on the surface to a level where intimate (molecular level) contact of the adhesive with the surface is achieved. Anything that gets in the way of this contact is a contaminant that must be removed or reduced to a non-threatening level by means of any number of cleaning techniques.
- Activation: The clean surface must be chemically active enough to form primary or secondary chemical bonds with the adhesive. A clean surface that is chemically inert cannot form the chemical bonds necessary for strong and reliable structural adhesion.
- Stabilization: The surface must be resistant to degradation (usually this means oxidation) when exposed to the service environment. The cleanliness and chemical activity of the surface needs to be maintained until the actual bonding or coating operation takes place.
The relative importance of these three aspects of surface treatment depends on the class of material under consideration, according to Dillingham. For example, metals have very high surface energies, meaning the surfaces are highly chemically reactive and contaminate quickly. Surface treatment for metals focuses on cleaning and creating a stable oxide. For composite materials, a different approach is needed for successful bonding and coating, because thermoset and thermoplastic polymers have relatively low surface energies and, therefore, do not contaminate as readily as metals and are relatively stable during environmental exposure. However, these same characteristics make adhesives less likely to stick to composites. As a result, surface treatment of composites usually focuses on the second factor listed above: increasing the surface energy so that a strong bond can be formed with an adhesive.
Determining surface energy

Manual abrasion is a common method of surface preparation for composites, but it may not be effective at cleaning a surface on its own.
Though generally low, surface energies can vary in different materials and composite parts, and surface treatments vary accordingly. According to Dillingham, the ability to quickly and quantitatively gauge the surface energy of an object or material is the important first step to designing, implementing or understanding the correct surface treatment.
There are several approaches to testing surface energy; one popular technique that BTG Labs often uses involves measuring the contact angle formed by a drop of fluid onto the test surface. In this method, if the liquid beads up upon contact with the surface, this indicates that it is not being attracted to the surface. Likely, an adhesive or paint will not be strongly attracted to this surface, either, and adhesion will be poor. Contamination is one cause for a surface to repel a liquid drop in this way.
However, if the liquid readily spreads out rather than beads up, this indicates that the surface is attracting the liquid strongly. Such a surface has high chemical energy and will, in general, adhere well to an adhesive. Dillingham notes that contamination with a surfactant, such as soap, will also cause liquids to spread on a surface, but that surfactant-induced wetting can be readily distinguished by the rate at which the liquid spreads.
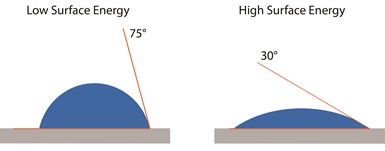
A water contact angle can be measured to indicate how bondable a surface is. A high contact angle (when the water drop beads up) usually indicates an insufficiently clean surface, and a low contact angle (when the water drop spreads out) indicates a properly cleaned surface.
The angle between a liquid drop and the surface — in other words, the contact angle (see image at left) — puts a value on the attraction of the surface for the liquid. There are several factors that determine what the target contact angle should be for a good adhesive bond on a given surface, including whether adhesion is being evaluated via a lap shear joint or a double cantilever beam (DCB). Generally, low contact angles (from 0 degrees to ~30-40 degrees) indicate a clean, high-energy surface that will establish good adhesion to adhesives and paints; high angles (60-90 degrees or more) indicate a low-energy or contaminated surface that will generally be difficult to bond to. A contact angle in the 40-60 degree range is less clear-cut: this can indicate a surface that is less predictably clean and ready for bonding than that with a lower contact angle, but that isn’t as certain to create weak bonds as a surface producing a contact angle measurements above that range.
Thermosets vs. thermoplastics
Thermoset composites (such as epoxies, polyimides, bismaleimides) and thermoplastic composites (such as PAEK, PEEK, PEKK and polyphenylene sulfide) have different surface characteristics and require different surface preparation strategies.
In some cases, Dillingham says, thermoset resins can benefit from surfacing films designed to increase the chemical reactivity of the composite surface. These surfaces typically show water contact angles in the 30-degree range after peel ply removal and are usually bondable. In other cases where the polymer surface is particularly unreactive, water contact angles are around 50-60 degrees, and surface treatments may be necessary for good adhesion.
Another surface treatment technique that has had some success with thermoset composites is abrasion, performed manually or via grit blasting. According to Dillingham, abrasion works because thermoset matrix resins are brittle polymers that fracture under abrasion by actual breaking of the polymer chains to create a chemically active surface. This surface can react with an adhesive to form a strong, stable interface. Depending on the chemical composition of the thermoset polymer, abrasion can reduce the water contact angle by 10 degrees or more, which can be sufficient for good bonding.

Lap joint strength vs. contact angle for PEKK bonded with Solvay 377S film adhesive
However, thermoplastic polymers behave differently from thermoset polymers. Because the polymer chains are not locked into a rigid network by crosslinking, Dillingham says, they tend to flow — in other words, deform plastically — under abrasion, rather than fracture. While an abraded thermoplastic composite may be rough, it is still chemically unreactive and unable to establish a good bond with an adhesive, coating or sealant. In addition, water contact angles on these surfaces generally do not change significantly with abrasion. For thermoplastic composites, plasma treatments can be an effective method for increasing surface energies. The figure above shows lap joint strength (vertical axis) versus contact angle (horizontal axis) for PEKK bonded with Solvay 377S film adhesive. According to the data, solvent wiping, hand sanding and grit blasting did not improve joint strength in this case, while plasma treatments increased strength by >30%. Furthermore, the plasma-treated samples failed cohesively in the adhesive, whereas the other samples failed at least partially interfacially between the adhesive and the substrate.
Strong, reliable adhesive bonds suitable for structural purposes are achievable between most structural materials, Dillingham concludes. However, surface treatments that work well for one class of material may not be appropriate for another; surface treatments need to be engineered with the specific chemical characteristics of the substrate and adhesive in mind. Most applications for thermoplastic composites require treatments that increase surface energy to an even higher extent than thermoset composites, so surface treatments should be treated differently. Combining surface treatments with appropriate measurement and control strategies ensures that surface treatments are effective and reliable.
Related Content
Plant tour: Albany Engineered Composites, Rochester, N.H., U.S.
Efficient, high-quality, well-controlled composites manufacturing at volume is the mantra for this 3D weaving specialist.
Read MoreSulapac introduces Sulapac Flow 1.7 to replace PLA, ABS and PP in FDM, FGF
Available as filament and granules for extrusion, new wood composite matches properties yet is compostable, eliminates microplastics and reduces carbon footprint.
Read MorePultrusion: The basics
A primer describing what pultrusion is, its advantages and disadvantages, and typical applications.
Read MoreTU Munich develops cuboidal conformable tanks using carbon fiber composites for increased hydrogen storage
Flat tank enabling standard platform for BEV and FCEV uses thermoplastic and thermoset composites, overwrapped skeleton design in pursuit of 25% more H2 storage.
Read MoreRead Next
BTG Labs launches Surface Analyst XA
The XA automates technology employed by the original handheld Surface Analyst unit, increasing the speed and efficiency of surface inspections.
Read MorePlant tour: Daher Shap’in TechCenter and composites production plant, Saint-Aignan-de-Grandlieu, France
Co-located R&D and production advance OOA thermosets, thermoplastics, welding, recycling and digital technologies for faster processing and certification of lighter, more sustainable composites.
Read More

.jpg;width=70;height=70;mode=crop)









.jpg;maxWidth=300;quality=90)