Medical applications: A healthy market
Composites make advances in devices for medical diagnosis and treatments that promote healing and help return patients to active lives.
Strapped into an Air Chair hydrofoil water ski, “Robert” raced through a slalom course on Utah’s Lake Powell. As he veered across the tow boat’s wake, his increased speed caused slack in the tow rope, and then, the unthinkable: “I reached out with my left hand to push the rope away from me to avoid becoming entangled,” he recalls. “Suddenly, I found myself dragged underwater with my left arm entangled in the rope.“ Unable to release himself from the Air Chair as he was dragged through the water. He felt the rope tighten around his arm until his left hand was severed from his arm below the wrist.
Robert survived, but as this avid sportsman pictured a future without water sports, skiing, snowboarding, golf and bowling, he assumed his life was over.
Composites lend a hand
Robert now has more than one hand to replace the one he lost, thanks to premier prosthetics manufacturer Otto Bock (Berlin, Germany and Minneapolis, Minn.). Otto Bock offers a range of technically exceptional prosthetics, including a variety of hands designed for specific activities. Robert has a personalized hand for golf and a general-purpose hand — and options for other special-purpose devices.
Although the hand devices are metal, the wraparound “soft socket” that encases the wearer’s forearm and provides the attachment point for prostheses is, in part, a composite. Otto Bock’s technical support manager, Jerry Gohman, explains that the sockets are made from cast impressions or digital imaging data gathered for each individual. Made from a variety of materials — flexible thermoplastics or thermoset laminates made by wet layup and reinforced with glass, hybrid glass/nylon or carbon fibers — the sockets end in an extension that accepts the prosthetic hand. Each extension is custom-made, typically a fiberglass-reinforced laminate, but sometimes combined with carbon fiber to accommodate the wearer’s size and activities.
Partial Hand Solutions (Southington, Conn.) has taken composites a step further, with the assistance of Canadian firm Vanguard Plastics (Surrey, British Columbia), by producing composite prosthetic fingers called M-Fingers, using RTP Company’s (Winona, Minn.) RTP 2300 Series glass-filled, rigid thermoplastic polyurethane to provide strength and dimensional stability for the inner structures of the fingers and multiposition thumb. Each inner structure is overmolded with RTP 1200 Series thermoplastic polyurethane elastomer, which gives each finger the dexterity to independently and gently conform to whatever it grasps.
Carbon fiber for fleet feet
The loss of a limb from an accident, war or disease is more frequent than one might think. Prosthetics and related orthotics (body part braces) together make up a $2.8 billion global market. The market for prosthetic feet in the U.S. alone is estimated at 300,000 units per year, according to Ron Nelson of ClosedMold Composites LLC (CMC, Salt Lake City, Utah). Demand for foot prosthetics has built a market for carbon fiber, which provides more energy storage and return (known as dynamic response) than any other material.
The dynamic response depends on springs that absorb energy during the heel-strike phase of a normal walking gait, and return it on the toe-off phase. Most foot prostheses today are based on a leaf-spring configuration. However, Nelson has invented the trademarked SpringWalk (pat. pend.), a prosthetic foot based instead on tightly coiled, hollow tubular springs. SpringWalk was developed and is marketed by Salt Lake City-based ClosedMold Carbon SpringWalk, a new firm formed by CMC. Nelson says the SpringWalk tubular structural elements transmit bending, torsional and shear loads very efficiently through the length of the foot’s spring elements in the direction, or orientation, of the carbon fiber. During the rollover phase between heel-strike and toe-off, the SpringWalk design functions integrally as a vertical shock absorber.
Since the tube centerline is curved in more than one plane — a spiraling coil vs. a flat U- or C-coil — it follows a tight curve, increasing the length of the energy-storing spring, thus increasing compliance. “The longer spring element, compared to a leaf spring, reduces stresses and softens the impact,” Nelson explains.
SpringWalk tubing is made using a proprietary combination of bladder molding and resin transfer molding, a process Nelson has patented and licensed for other commercial composite products.
SpringWalk is not yet on the market but amputee trials show promise and the semi-automated molding process could make it price-competitive.
Composite body implants
Composites also are making headway in implantable medical devices. Historically, implant designers have faced difficulty achieving acceptance from regulatory agencies. Nevertheless, the use of composite implants is on the rise. New composite materials and medical applications for them are gaining approval in greater numbers from the U.S. Food and Drug Admin. (FDA, Silver Spring, Md.) and the European Commission (Brussels, Belgium), whose “CE” marking certifies that a product meets European Union consumer use standards.
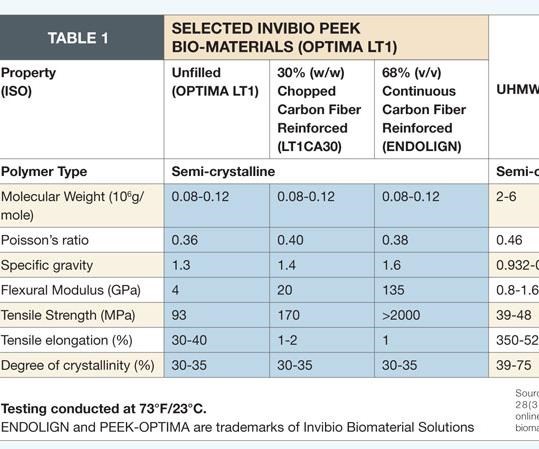
PolyMedex Discovery Group (Putnam, Conn.) recently introduced bone growth formulations for implants, such as bone screws and temporary bone supports used in orthopedic repairs. Applications include bioresorbable polymers, which break down and can be safely absorbed by the body as the body rebuilds the bone. Bioresorbables such as polycaprolactone (PCL), polylactide (PLA) and polyglycolide (PGA) are customized for absorption periods, ranging from 1 to 36 months and flexural modulus (stiffness) from 30,000 psi (200 MPa) to more than 1,000,000 psi (6,900 MPa). The company also has non-bioresorbable polymers for direct bone replacement, to fill a void in bones damaged beyond self-repair.
Implantable polymers, such as polyetheretherketone (PEEK), have been used for some 20 years in permanent implants, often in high-stress applications. For example, ENDOLIGN continuous carbon fiber/PEEK from the Invibio Biomaterial Solutions division of Victrex USA Inc. (W. Conshohocken, Pa. and Lancashire, U.K.) is used as an alternative to metals in the development of implantable load-bearing components for orthopedic applications.(ENDOLIGN and Invibio’s PEEK-OPTIMA polymer, another implantable biomaterial, are trademarks of Invibio Biomaterial Solutions.)
Although load-bearing implants must be stiff enough to support a fractured or otherwise unstable bone, their purpose is best served by sharing the load with the original bone, rather than shielding the bone from stress. Stress-sharing is in accordance with Julius Wolff’s Law of Bone Adaptation (1892), which established that repeated mechanical stress on a bone results in modeling and remodeling — and thus better maintenance — of the bone. The optimal implant is, therefore, strong but somewhat flexible, with a modulus of elasticity as close as possible to that of the bone. New polymeric and composite materials are under development to fulfill this requirement and are increasingly acknowledged as better alternatives to stiff, rigid metal devices.
Composite bone implant approved
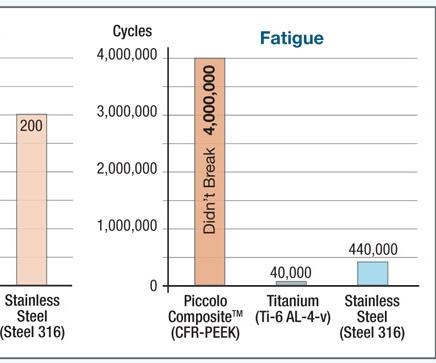
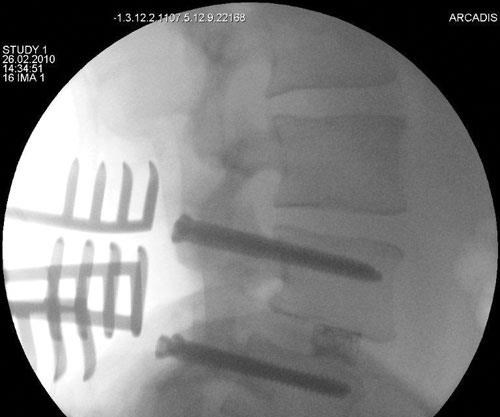
NMB Medical Applications (Herzeliya, Israel) received the EU’s CE marking in 2009 and obtained FDA clearance in 2010 for its Piccolo Humeral Composite Nailing System. Piccolo is an intramedullary (inside-the-bone) system for treatment of long-bone (e.g., humerus, femur or tibia) fractures. Carbon fiber/PEEK rods (hence, the name “nails”) are inserted into the bone’s medullary (central marrow) cavity to align and stabilize the fracture. As noted above, the nails share the load with the bone, promote healing and permit the patient to begin using the extremity again more quickly. The nail is made using longitudinal and bidirectional helical carbon fibers in a PEEK matrix. The result is enhanced biomechanical properties and a modulus of elasticity that is very similar to that of cortical bone. Additionally, the materials render the nail radiolucent and completely compatible with x-ray, computerized tomography (CT) and magnetic resonance imaging (MRI) scans, enabling better post-operative monitoring of the fracture site.
Composite lumbar spacer implant
Another breakthrough is the composite ETurn Ti lumbar spacer implant developed by icotec AG (Altstätten, Switzerland). Icotec received CE approval in January 2010, which allowed for marketing and implantation in the European market. ETurn Ti is designed for posterior intersomatic spondylodesis, a surgical procedure for spinal disk replacement. Ronald Wieling, vice president of Icotec Medical, explains, “The disk material is removed and replaced with the spacer. The function of the spacer is the restoration of the height of the collapsed disc space and the stabilization of the adjacent vertebral elements to enable bone fusion. Restoration of the disk height releases the pressure on the nerve root.”
The ETurn Ti is made using carbon fiber-reinforced PEEK, with additional reinforcement from tantalum fibers. Tantalum, a heavy metal, makes the otherwise translucent spacer visible during MRI imaging. “The translucent [composite] material enables the surgeon to judge the quality of the bone fusion [while] the tantalum markers allow for post-operative implant position monitoring,” Wieling says.
The spacer also is coated with titanium to improve the implant/bone interface. “Normally the bone doesn’t grow directly onto PEEK. The titanium coating ensures a very direct contact between the bone and implant,” Wieling says, claiming, “This combination of CF PEEK with the titanium coating ... is unique for this kind of implant. Icotec is the only company providing such products.”
Icotec manufactures the spacer, fastening implant elements and other load-bearing components using its Composite Flow Molding (CFM) process, in which pultruded carbon fiber/thermoplastic rods (50 to 60 percent fiber by volume) are heated above the matrix melt temperature, then forced into a mold cavity during a proprietary injection molding process. Wieling adds, “The special thing about our process is the way we bring the material into the mold.” The method permits the use of long fibers at high volume fractions, significantly boosting mechanical properties compared to low-volume, chopped-fiber products.
Surgical seals
During some surgical procedures, the body must be protected from lubricants used in bone saws, drills, and other powered hand tools now available to surgeons. Similarly, the tools’ inner workings must be protected from bodily fluids and debris.
Bal Seal Engineering (Foothill Ranch, Calif.) custom designs spring-energized sealing solutions for the medical industry. “The feedback we’ve received from our customers is that composite seals ... offer several advantages over elastomer seals and other popular alternatives,” says Bal Seal’s global medical device market manager Steve Twork.
Bal Seal custom blends its own materials. In the past, its seals for surgical applications used carbon fiber-filled polytetrafluoroethylene (PTFE), but Twork says OEMs increasingly request other composite blends. Bal Seal most often recommends its SP45, a proprietary blend of PTFE and polymer fiber to meet the hardware requirements of soft metals by minimizing seal-to-shaft damage. This formula eliminates the abrasiveness of carbon, reducing the wear factor by as much as 30 percent. But SP45 is intended for low pressure and high speeds. “If the application were high pressure and low speed, I would suggest the carbon-filled PTFE materials,” Twork clarifies.
SP45 also has high-temperature tolerance. “Elastomers don’t hold up well in a [medical] autoclave, and that’s a very important consideration,” Twork points out. Depending on the formulation, he says, surgical tools with composite seals can endure hundreds of autoclave sterilization cycles at temperatures from 270°F/132°C to as high as 475°F/246°C, yet still hit performance targets.
See-through supports
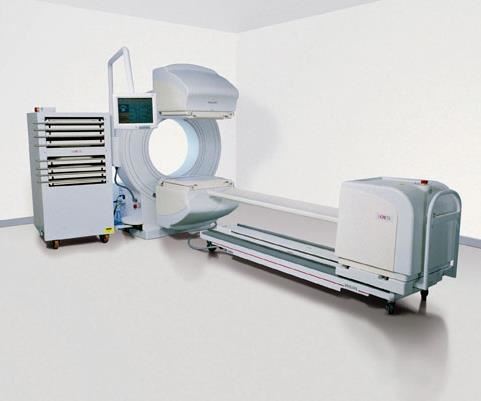
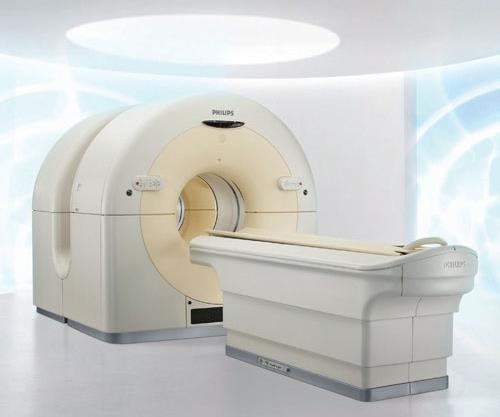
Carbon fiber composites have found a strong market in patient imaging tables and accessories used in nuclear (X-ray), CT, positron emission tomography (PET) and MRI systems. Carbon composites are radiolucent — that is, they absorb very low levels of radiant energy, minimizing signal attenuation to enable capture of clear images of target body parts. Carbon structures also are strong enough to support the patient in a cantilever- or gantry-type system that has no intermediate supports. The challenge, then, is to achieve adequate strength yet minimize mass, to ensure the least possible signal interference.
Composites Horizons Inc. (CHI, Covina, Calif.) manufactures radiolucent composite tables and other support equipment for imaging systems and surgical tables that allow surgeons a clear real-time view of an ongoing surgical procedure. CHI lays up carbon/epoxy prepreg and cocures it in a proprietary process with a core such as ROHACELL polymethacrylimide (PMI) structural foam from Evonik Foams Inc. - ROHACELL (Darmstadt, Germany). Because of its high strength, only a relatively thin layer of PMI foam is required for the tables. Carbon 12K and 6K tow are used, and the fiber volume is greater than 50 percent.
Healthy choices
Despite a stagnant economy, health care is thriving and new composites regularly enter the market. The current challenge is regulatory. Carbon-fiber/PEEK implants, for example, have been used for some 20 years, but the FDA has yet to issue overall regulations for composite implants. Devices that incorporate new materials, therefore, still face long, stringent, case-by-case approval processes. Even so, composites are making headway, especially in bone implants. More than 1.5 million people need treatment for fragility fractures annually, according to Dr. Samir Mehta, speaking at the 2010 Annual Meeting of the American Academy of Orthopedic Surgeons. Mehta expects an “epidemic” of fragility fractures as 78 million baby boomers retire.
In the field of prosthetics, carbon composites are so effective that in some open running competitions, carbon composite foot prosthetics have been disqualified due to the advantages they give the “disabled” runner.
In prosthetics, implant surgery and diagnostics, composites continue to gain recognition as the healthy choice.
Related Content
Combining multifunctional thermoplastic composites, additive manufacturing for next-gen airframe structures
The DOMMINIO project combines AFP with 3D printed gyroid cores, embedded SHM sensors and smart materials for induction-driven disassembly of parts at end of life.
Read MoreWelding is not bonding
Discussion of the issues in our understanding of thermoplastic composite welded structures and certification of the latest materials and welding technologies for future airframes.
Read MorePEEK vs. PEKK vs. PAEK and continuous compression molding
Suppliers of thermoplastics and carbon fiber chime in regarding PEEK vs. PEKK, and now PAEK, as well as in-situ consolidation — the supply chain for thermoplastic tape composites continues to evolve.
Read MoreDeveloping repairs for thermoplastic composite aerostructures
HyPatchRepair project proves feasibility of automated process chain for welded thermoplastic composite patch repairs.
Read MoreRead Next
Plant tour: Daher Shap’in TechCenter and composites production plant, Saint-Aignan-de-Grandlieu, France
Co-located R&D and production advance OOA thermosets, thermoplastics, welding, recycling and digital technologies for faster processing and certification of lighter, more sustainable composites.
Read MoreAll-recycled, needle-punched nonwoven CFRP slashes carbon footprint of Formula 2 seat
Dallara and Tenowo collaborate to produce a race-ready Formula 2 seat using recycled carbon fiber, reducing CO2 emissions by 97.5% compared to virgin materials.
Read MoreVIDEO: High-volume processing for fiberglass components
Cannon Ergos, a company specializing in high-ton presses and equipment for composites fabrication and plastics processing, displayed automotive and industrial components at CAMX 2024.
Read More















.jpg;maxWidth=300;quality=90)