KIT produces graphene from carbon dioxide
University researchers have developed a method for direct synthesis of graphene from CO2, targeting potential applications in batteries and electronics.
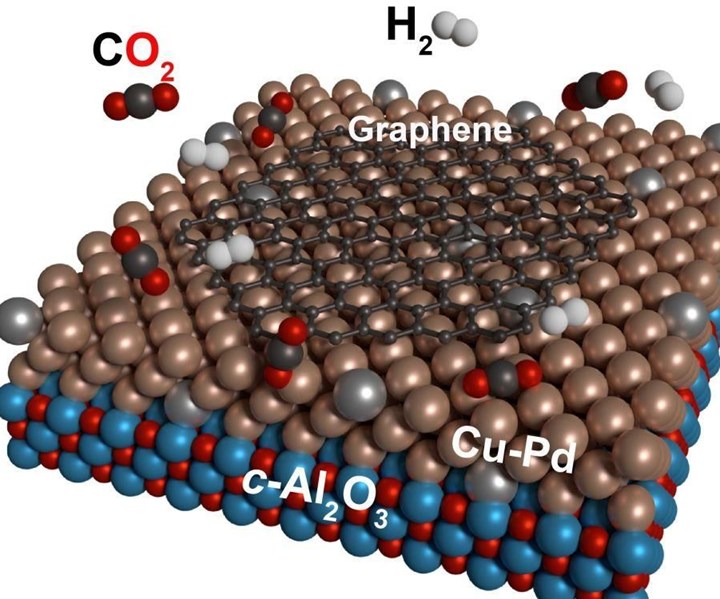
Carbon dioxide (red-black) and hydrogen (gray) react catalytically on copper-palladium surfaces to form the technology material graphene (black). Source | E. Moreno-Pineda at KIT, via Springer lightweight.design magazine
A working group of the Karlsruhe Institute of Technology (KIT; Karlsruhe, Germany) is using carbon dioxide as a starting material to produce graphene, according to the researchers’ report in the journal ChemSusChem.
The scientists say they have developed a process in which the greenhouse gas carbon dioxide (CO2), together with hydrogen, is directly transferred into the technology material graphene with the aid of specially prepared, catalytically active metal surfaces at temperatures of up to 1,000°C.
“If the metal surface has the right balance of copper and palladium, the conversion of carbon dioxide to graphene will take place directly in a simple one-step process,” says study leader Professor Mario Ruben of the Molecular Materials Working Group at the Institute of Nanotechnology (INT; Evanston, Ill., U.S.) and at the Institute of Inorganic Chemistry (AOC) of KIT.
In further experiments, the researchers produced the graphene with several layers of thickness, targeting possible applications in batteries, electronic components or filter materials. The next research goal of the working group is to shape functioning electronic components from the graphene obtained.
Graphene is the two-dimensional form of the chemical element carbon, which has electrical properties and enables powerful batteries. Learn more about graphene and other nanomaterials, and their use in nanocomposites, in “Nanomaterials: Products, supply chain mature for next-gen composites.”
This post is courtesy of the CompositesWorld and Springer lightweight.design magazine media partnership. For more information about Springer and lightweight.design, go to https://www.springerprofessional.de/en/link/12141380
Related Content
-
Plant tour: Joby Aviation, Marina, Calif., U.S.
As the advanced air mobility market begins to take shape, market leader Joby Aviation works to industrialize composites manufacturing for its first-generation, composites-intensive, all-electric air taxi.
-
PEEK vs. PEKK vs. PAEK and continuous compression molding
Suppliers of thermoplastics and carbon fiber chime in regarding PEEK vs. PEKK, and now PAEK, as well as in-situ consolidation — the supply chain for thermoplastic tape composites continues to evolve.
-
Bio-based acrylonitrile for carbon fiber manufacture
The quest for a sustainable source of acrylonitrile for carbon fiber manufacture has made the leap from the lab to the market.















