Self-organization and photopolymerization method constructs tailorable 2D polymer materials
International research team uses fantrip molecule and a violet laser to create durable covalent bonds for ultra-thin, functional materials with highly defined and regular crystalline structures.
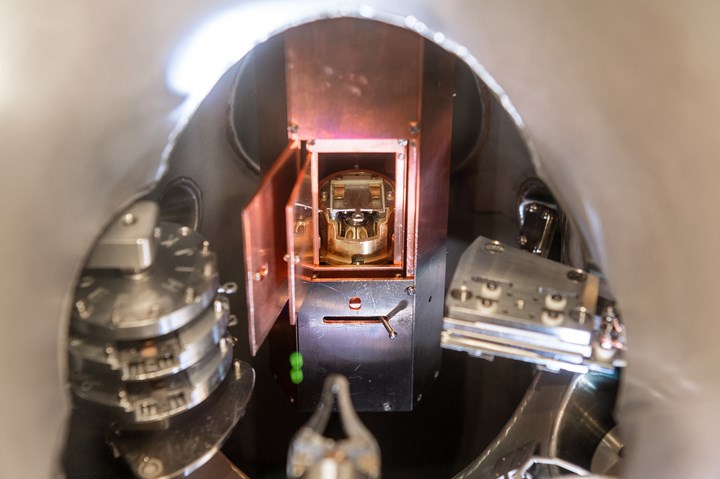
This is the ultra-high vacuum chamber in which the experiments, including the preparation and analysis of the samples, were carried out. The middle part shows the self-made low-temperature scanning tunneling microscope with an inserted sample. On the left is the carousel for storing the samples in a vacuum. The sample heater for preparing the samples in a vacuum is located on the right-hand side. In the lower center are the tongs of the vacuum gripper, ie the “hand” for transferring the samples between the different stations. Photo Credit: Andreas Heddergott/TUM
An international research team1, led by members from the Technical University of Munich (Germany), the Deutsches Museum and the Linköping University (Sweden), has developed a method to manufacture 2D polymers with the thickness of a single molecule. The polymers are formed on a surface by the action of light. The discovery is said to make it possible to develop new ultra-thin, functional materials with highly defined and regular crystalline structures.
Two main approaches are used to create ultra-thin materials, the research team explains. In the first, a continuous layer of molecules or atoms is “peeled off” from the bulk of the material. Graphene is an example of such a material.
The other approach, in contrast, involves the construction of the material molecule-by-molecule by producing bonds between the molecules in various ways. Researchers note that the problem is that the materials are often small, fragile and contain many defects. This limits the potential areas of application.
The manufacture, or polymerization, of these new 2D polymers takes place in two steps. The researchers use a molecule known as “fantrip.” Fantrip is a contraction of “fluorinated anthracene triptycene.” This molecule is a merger of two different hydrocarbons — anthracene and triptycene. The specific properties of fantrip cause the molecules to spontaneously arrange themselves into a pattern when they are placed onto a graphite surface covered with an alkane. This process is known as “self-organization.”
The next step is the photopolymerization itself, when the pattern is to be fixed with the aid of light. Taking place in a vacuum (which ensures that the material is not contaminated), the molecules are illuminated by a violet laser that excites the electrons in the outermost electron shell. This causes strong and durable covalent bonds to form between the molecules.
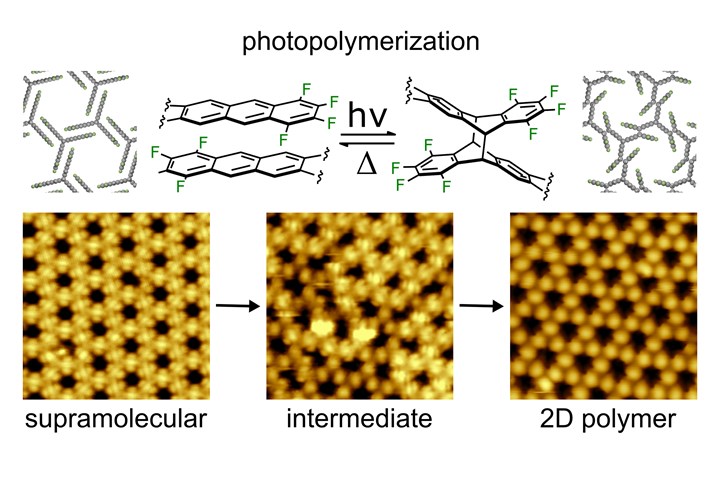
Reaction path from the self-organized molecular structure to the regularly linked 2D polymer. Photo Credit: Markus Lackinger/TUM
“Creating covalent bonds between molecules requires a lot of energy. The most common way of supplying energy is to raise the temperature, but this also causes the molecules to start moving,” says Markus Lackinger, research group leader at the Deutsches Museum and Technical University of Munich. “So it won’t work with self-organized molecules, since the pattern would blur. Using light to create covalent bonds preserves the pattern and fixes it precisely as we want it.”
The result is a porous 2D polymer, half a nanometer thick, consisting of several hundred thousand molecules identically linked; in other words, the team says, a material with nearly perfect order, right down to the atomic level.
Further, since the photopolymerization is carried out on a surface of solid graphite, it is also possible to follow the process on the molecular scale using scanning tunneling microscopy. This shows the newly formed bonds in a persistent network. In order to confirm the structure assignment, the research group, led by Jonas Björk, has simulated the appearance of the molecular networks under the microscope at different stages of the reaction.
Jonas Björk is assistant professor in the Materials Design Division at the Department of Physics, Chemistry and Biology at Linköping University. He has used high-performance computing resources at the National Supercomputer Centre in Linköping to validate the experiments and understand the key factors that make the method successful.
“We see that the simulations agree well with reality down to the tiniest detail, and we can also understand why our specific system gives such useful results,” Björk says. “The next step of the research will be to see whether the method can be used to link other molecules for new 2D and functional materials. By improving the method, we will also be able to control and tailor the type of ultra-thin materials we aim to manufacture.”
Lackinger notes that, because the final 2D polymer film is also stable under atmospheric conditions, the material will find many conceivable applications. “The most obvious application is to use the material as filter or membrane, but applications that we have no idea of at the moment in entirely different contexts may appear on the horizon, also by chance. This is why basic research is so exciting,” says Lackinger.
1Lukas Grossmann, Benjamin T. King, Stefan Reichlmaier, Nicolai Hartmann, Johanna Rosen, Wolfgang M. Heckl, Jonas Björk, Markus Lackinger
On-surface photopolymerization of two-dimensional polymers ordered on the mesoscale
Nature Chemistry, June 3rd, 2021 – DOI: 10.1038/s41557-021-00709-y
https://www.nature.com/articles/s41557-021-00709-y
Read Next
Plant tour: Daher Shap’in TechCenter and composites production plant, Saint-Aignan-de-Grandlieu, France
Co-located R&D and production advance OOA thermosets, thermoplastics, welding, recycling and digital technologies for faster processing and certification of lighter, more sustainable composites.
Read MoreDeveloping bonded composite repair for ships, offshore units
Bureau Veritas and industry partners issue guidelines and pave the way for certification via StrengthBond Offshore project.
Read MoreAll-recycled, needle-punched nonwoven CFRP slashes carbon footprint of Formula 2 seat
Dallara and Tenowo collaborate to produce a race-ready Formula 2 seat using recycled carbon fiber, reducing CO2 emissions by 97.5% compared to virgin materials.
Read More




















