Uncompromising composite hyperbaric oxygen chamber closes the gap
Flexible filament-wound composite reduce weight and cost, increase performance of first portable high-pressure oxygen-delivery system.
Design Results:
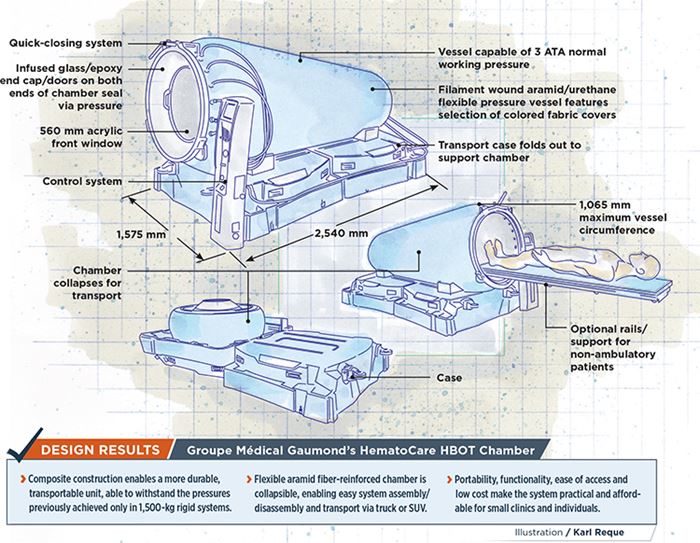
- Composite construction enables a more durable, transportable unit, able to withstand the pressures previously achieved only in 1,500-kg rigid systems.
- Flexible aramid fiber-reinforced chamber is collapsible, enabling easy system assembly/disassembly and transport via truck or SUV.
- Portability, functionality, ease of access and low cost make the system practical and affordable for small clinics and individuals.
An established medical procedure, hyperbaric oxygen therapy (HBOT), temporarily delivers pure oxygen to a patient under higher-than-atmospheric pressure, enabling blood to carry more oxygen, which, in turn, helps fight infection, stimulates release of growth factors and stem cells and speeds healing. Most notably used to treat divers afflicted with decompression sickness (the “bends”), it’s employed routinely to treat altitude sickness, severe anemia, carbon monoxide poisoning and many other medical conditions — and to enhance the performance of elite athletes.
HBOT requires a hyperbaric chamber. Large multi-person units resemble hospital rooms, where patients receive oxygen via facemask or clear hood while sitting or lying down. Much smaller, single-person systems come in rigid and flexible formats, and treat patients as they recline inside a narrow tubular chamber. Rigid (hard-shelled) systems can reach pressures up to 3 atmosphere absolute (ATA; that is, three times normal atmospheric pressure) and weigh up to 1,500 kg. Permanent installations, they typically are constructed of metal, with entry hatches/airlock doors, glass or acrylic observation windows or closed-circuit TV, plus two-way communications for patient monitoring. Considerably heavier, bulkier and costlier to build and operate than flexible units, most are owned/operated by naval or diving organizations, hospitals and dedicated recompression facilities. Flexible systems operate at much lower pressures (to 1.4 ATA) and don’t provide the same efficacy or meet the same performance requirements as rigid chambers, but they are usually transportable because they are constructed of lightweight urethane- or vinyl-coated nylon-bonded fabric and can be collapsed. Patients enter through a full-length, double-zipper seal from the top of the chamber, which is pressurized with oxygen-enriched compressed air.
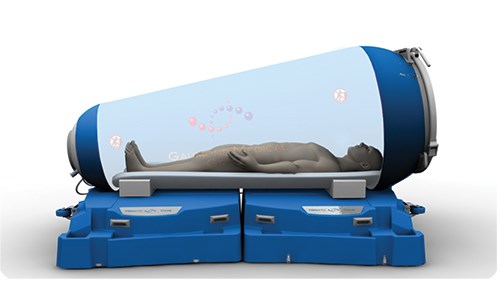
Now, the HematoCare, a new hyperbaric chamber from Groupe Médical Gaumond (GMG, Montréal, QC, Canada), is set to close the gap between rigid and flexible single-person systems. Unique composite construction enables the chamber to deliver oxygen at the high pressures of rigid systems, but keep system cost, weight and collapsed size down for maximum medical utility, convenient stowage and portability, and rapid deployment.
A decade in development
Claude Gaumond, GMG’s CEO, made a decidedly untraditional transition into the medical-device field. An elite cross-country skier and triathlete, his initial interest in HBOT was to enhance his own competitive performance. However, while exploring available units in 2001, he recognized that each had shortcomings, and he started wondering how to design a better system. During his university business studies, he took the opportunity to use his idea as a business case in his classes. As a graduate, he had a good idea and a solid business plan, but limited funds and little technical knowledge about how to engineer such a complex product. Here, an early and ongoing resource was the National Research Council Canada’s (NRCC/CNRC) Industrial Research Assistance Program (IRAP, Ottawa, ON, Canada), which in 2005 provided funding and connected him with specialists in the regulatory, electronics, engineering and composites arenas.
From the start, Gaumond’s team wanted to build a low-cost, flexible unit that could match the 3.0 ATA pressures of rigid hyperbaric chambers. This was a tall order: no other soft-sided system had yet been rated higher than 1.4 ATA, because to be certified by the US Food & Drug Admin. (FDA) at a maximum allowable working pressure (MAWP), devices are tested at six times that pressure.
Another challenge was that the chamber needed to be sufficiently pliable to fold and store compactly, and light enough to move around but also strong enough to withstand pressure differentials without tearing during normal use. While benchmarking fabrics from existing systems, Gaumond’s researchers realized these membranes would never survive such high pressures without leaking, so something new would be needed.
Also, many HBOT patients have impaired ambulation so existing flexible designs that required them to enter from the top were judged clumsy and undesirable. That led to brainstorming about ingress/egress options that would permit efficient chamber sealing, yet keep the system lightweight and inexpensive. The team took its cues from rigid units, which have doors in the horizontal chamber ends. That necessitated finding new ways to meet cost, mass and pressure targets. GMG also wanted to make the chamber as large in diameter as possible to accommodate larger patients (up to 220 kg). The largest flexible systems measured 102 cm in diameter, but the team’s target was 107 cm. (As diameter increases, thermodynamic requirements in pressure vessels increase much faster.) Durability was another important criterion (the goal was 10 years/4,000 cycles), and Gaumond was adamant: he wanted something as good-looking as it was functional. Citing former Apple Inc. CEO Steve Jobs’ influence on his design sensibility, Gaumond recalls, “I told my team in the beginning, ‘You make the system good, and I will make it beautiful.’”
Another goal was global sales, so the design had to accommodate multiple electrical systems and receptacles and meet challenging hyperbaric standards worldwide, preferably with one design to keep costs down. That committed team members to expensive and time-consuming rounds of testing in each target region.
Developing the system
A major obstacle was developing a flexible membrane capable of meeting the application’s mechanical performance, softness (Shore D 25) and stringent self-extinguishing flame-retardancy requirements. Given his elite athletic background, Gaumond was accustomed to paying a premium price for premium performance and associated that equation with composites, so the team began with conventional glass-reinforced vinyl, but it quickly failed. Likewise, no zipper systems withstood high pressures without leaks. In 2007, a French professor of textiles joined the team. Representatives from Cytec Industries Inc. (Woodland Park, NJ, US) and aerospace/defense composites molder, Stelia Aerospace North America (Lunenburg, NS, Canada), also came aboard. After much research and testing, the material that finally worked was a high-performance aramid fiber from Teijin Ltd. (Osaka, Japan), impregnated with a custom polyurethane formulation. Carbon fiber had proved too stiff and glass fiber too heavy. Aramid was light, tenacious, flexible and flame retardant. Many forming processes were considered, but filament winding was deemed best for the conical chamber. The commercial chambers are produced by Stelia.
To meet the ingress/egress challenge, the team developed round doors and windows at both chamber ends. The gel-coated, fiberglass-reinforced epoxy end caps are infused by GMG, which is now an ISO 13485-certified medical-device manufacturer. Post-mold finishing involves machining and insertion of an aluminum 6061-T6 support ring, which is bonded to the composite prior to acrylic-window insertion. A rubber O-ring and final clamping ring lock the curved window into the chamber side of the door. Cleverly designed, the caps fit snugly into the flexible membrane, yet are not permanently attached — when air is flowing into the chamber, pressure alone holds caps and windows tightly in place, preventing leaks. An optional stretcher-support add-on features two rails that run along each side of the chamber to assist attendants with patients too ill to enter on their own (lower right image in drawing, top left).
A tough but lightweight, two-piece transport case with casters (packed dimensions are 1,510 mm long by 725 mm wide by 1,200 mm tall) opens to form the support base for the 2,540 mm long by 1,575 mm wide by 1,420 mm tall deployed chamber. GMG rotomolds the cases, which are certified to hold 200 kg, from unreinforced high-density polyethylene.
At only 125 kg packed weight, it is the world’s lightest and least costly 3 ATA-certified hyperbaric chamber. Last year, the system won a JEC Innovation Award in the Medical category and an Alliance Monde Polymères prize.
Chasing global certification
Because HBOT is a medical procedure involving pure oxygen (a fire hazard) delivered inside a pressure vessel, it’s a heavily regulated device. Before a manufacturer can sell one, it must be certified. GMG has spent years in long and costly testing toward that end, and in fortifying its global patent portfolio. To date, HematoCare has been approved in many markets, including the prestigious CE Mark from the European Medical Device Directives. At press time, CW received word that it had received PVHO Committee Approval from the American Society of Mechanical Engineers, making GMG’s HematoCare the first transportable HBOT system rated to 3 ATA with global approvals. Distribution deals are in the works, and commercial sales are expected soon.
“Claude Gaumond has endured a lot of challenges developing this product, but has solved them one by one,” recalls team-member Claude Baril, Stelia managing director. “He has worked so hard and never stopped believing in his vision, like a true entrepreneur. What has been done here is quite an accomplishment.”
Related Content
Plant tour: Joby Aviation, Marina, Calif., U.S.
As the advanced air mobility market begins to take shape, market leader Joby Aviation works to industrialize composites manufacturing for its first-generation, composites-intensive, all-electric air taxi.
Read MoreThe next evolution in AFP
Automated fiber placement develops into more compact, flexible, modular and digitized systems with multi-material and process capabilities.
Read MoreLarge-format 3D printing enables toolless, rapid production for AUVs
Dive Technologies started by 3D printing prototypes of its composite autonomous underwater vehicles, but AM became the solution for customizable, toolless production.
Read MoreA new era for ceramic matrix composites
CMC is expanding, with new fiber production in Europe, faster processes and higher temperature materials enabling applications for industry, hypersonics and New Space.
Read MoreRead Next
CNG tanks: Pressure vessel epicenter
In the pressure vessel market, there are a number of competing designs offered around the world, including a small number of new CNG Type V “linerless” designs, which have recently received regulatory approval for use in vehicle applications.
Read MoreDesigning pressure vessels for seawater desalination plants
Safe high-pressure service challenges manufacturers of composite pressure vessels.
Read MorePressure vessels for alternative fuels, 2014-2023
Lower fuel costs and escalating emissions standards are driving a 10 percent annual growth in alternative fuel pressure vessel sales.
Read More
.jpg;width=70;height=70;mode=crop)